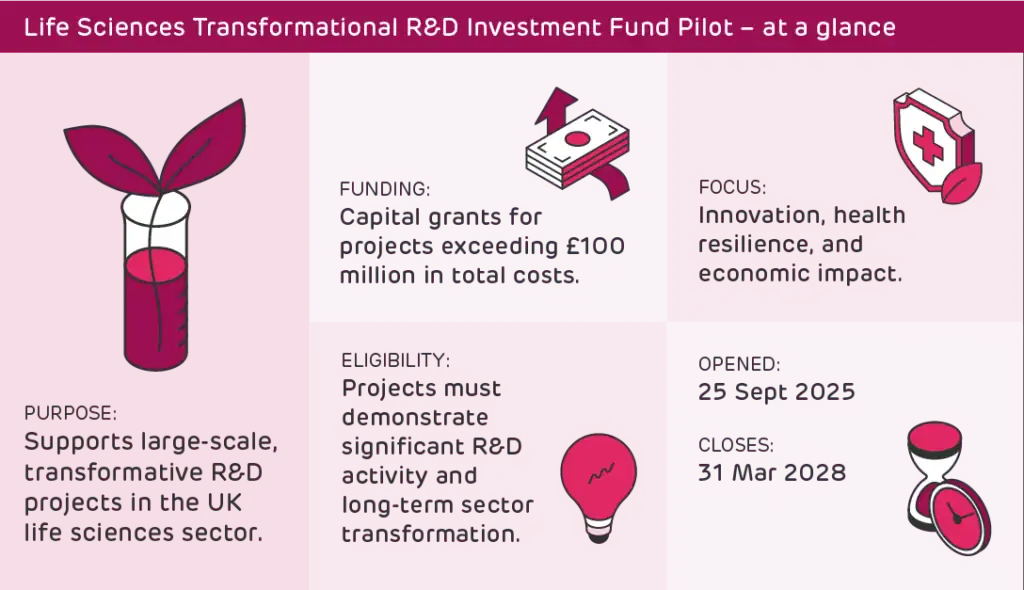
The UK’s £100 billion life sciences sector has been given a boost by a major new funding opportunity: the Life Sciences Transformational R&D Investment Fund (TRIF) Pilot. This is a £50 million capital grant opportunity run by the Department for Science, Innovation and Technology (DSIT), aimed at projects with a total cost (including capital expenditure) of at least £100 million.
ForrestBrown’s experienced grant advisory team can support with all aspects of the application process and subsequent due diligence. We can apply the practical insights and expertise gained from working on successful life sciences grant applications to your project, enabling you to submit your application with confidence.
What is the TRIF pilot?
The TRIF Pilot is a £50 million capital grant fund designed to support large-scale, transformative R&D-intensive investment projects in the UK life sciences sector. The fund aims to strengthen the UK’s health resilience and drive innovation, while also delivering significant economic benefit.
Which organisations are eligible for the TRIF Pilot?
The TRIF Pilot is focused on large-scale transformative R&D investments. The R&D project should demonstrate how it strengthens UK health resilience and drives innovation by either:
- Expanding R&D capacity in life sciences: such as drug development, medical devices, clinical research, or manufacturing efficiency.
- Improving flexibility in R&D infrastructure: enabling rapid scaling or redeployment during health emergencies through advanced technologies, AI, or accelerated testing and production.
Applicants must be a UK-based life sciences company operating as a:
- Product developer,
- Contract development and manufacturing organisation, or
- Generics manufacturer.
Which projects are eligible for the TRIF Pilot?
Eligible R&D projects must be focused within three areas:
- Human medicines: projects that focus on the development and innovation of pharmaceuticals.
- Medical diagnostics: projects aimed at advancing tools, systems, or methods for the identification and monitoring of diseases.
- MedTech products: creation and enhancement of medical devices specifically related to human health.
What are the fund’s objectives?
TRIF Pilot has two core objectives:
Health resilience and innovation.
Projects must support or demonstrate how they will strengthen this objective, either by:
- Creating new or expanding existing UK R&D capacity to support innovation in life sciences and ensure the UK is better equipped to respond to emerging healthcare challenges; or
- Enhancing flexibility in R&D facilities, capabilities and technologies, enabling rapid redeployment or scaling during health emergencies.
Creating economic opportunity.
This can take several forms:
- Generating R&D spillover benefits.
- Substantial contribution to gross value added.
- Creation of high-wage, high-skilled jobs.
When can I apply to the TRIF Pilot?
The TRIF Pilot opened on 25 September 2025 and will remain open until 31 March 2028, or until all the funding has been allocated.
Expressions of interest (EoIs) can be made via the online portal. Eligibility is then evaluated to ensure that projects align with the fund’s objectives. Successful applicants will be invited to the next stage, where the health resilience and innovation benefits are assessed by a panel, as well as project viability and deliverability.
Panels run monthly and for the remainder of 2025 are on:
- 17 November 2025.
- 15 December 2025.
Applicants that make it through the panel stage will then progress to a third phase, covering financial information, economic assessment and projects risks.
Benefits of the TRIF Pilot
TRIF Pilot offers a complementary scheme to the £520 million Life Sciences Innovative Manufacturing Fund (LSIMF), which supports projects with a minimum spend of £8 million.
While TRIF Pilot focuses on early-stage innovation and transformative R&D, the LSIMF supports the commercialisation and manufacturing of those innovations. Together, these funds offer support across the full spectrum of innovation, from early-stage R&D through to advanced manufacturing scale-up.
Organisations pursuing TRIF Pilot-funded R&D may later benefit from LSIMF to bring those innovations to market. Companies that have already received LSIMF funding but have new R&D projects that meet the criteria for TRIF Pilot, can apply.
How ForrestBrown can support your TRIF Pilot application
ForrestBrown’s experienced grants team, led by Karim Budabuss, can support you at any stage of the TRIF Pilot process. Whether you need eligibility assessment, financial modelling, project management or post-offer-in principle due diligence, we have the skills for you.
Our strong track record of securing funding for life sciences clients, includes successful applications to the LSIMF. We use the knowledge gained from those applications to refine future submissions, ensuring that applications are robust and meet the established criteria.
While we handle most applications from the outset, we can also progress applications that have been started in-house but require additional resource to complete. Similarly, if you’ve received an offer-in-principle, but need a new adviser to complete the due diligence, we can help.
Ready to start your TRIF Pilot application?
If you’re a firm in the life sciences sector that potentially meets the eligibility for TRIF, then get in touch to begin your application.
- Telephone
- 0117 926 9022
- hello@forrestbrown.co.uk
